[24][25] There have also been reports of abuse in Mashhad Central Prison in Iran. Concept and design: van Esch, van Zuylen, Geijteman, Oomen-de Hoop, Boogaard, van der Heide, van der Rijt. E.
Antacids and certain anti-diarrhea drugs (adsorbent-type drugs such as kaolin-pectin, attapulgite) lower the absorption of scopolamine.

The primary outcome was the occurrence of a grade 2 or higher death rattle as defined by Back (range, 0-3; 0, no rattle; 3, rattle audible standing in the door opening) measured at 2 consecutive time points with a 4-hour interval. Always ask your health care professional for complete information about this product and your specific health needs. Accessibility Statement, Our website uses cookies to enhance your experience. HJ. [9] Greater care is recommended in those with heart problems.
butylbromide scopolamine anthocyanin powder butylbromide scopolamine purity bromide rosmarinic cas triclosan extract rosemary leaf natural ol replace larger acid alibaba P,
2022 American Medical Association. Among all 157 patients, 86% had cancer as primary diagnosis. Mainly eliminated in the feces (69.7%) with very little in the urine (4.4%) 1. This document does not contain all possible drug interactions. Each box contained 16 identical ampoules of either scopolamine butylbromide (20 mg in 1 mL) or placebo (physiological saline 1 mL), which was sufficient for treating 1 patient for 4 days. Randomized double-blind trial of sublingual atropine vs placebo for the management of death rattle. Care for the dying, national guideline. S. The effect is to prevent spasms of the stomach, intestine or urinary bladder. Published 2010. Secondary outcomes included the time between recognizing the dying phase and the onset of a death rattle and anticholinergic adverse events.

When the dying phase was recognized, patients fulfilling the eligibility criteria were randomized. Scopolamine butylbromide binds to muscarinic M3 receptors in the gastrointestinal tract 1. Physical activity related questions will be answered by one of HealthLink BCs qualified exercise professionals. Among 162 patients who were randomized, 157 patients (97%; median age, 76 years [IQR, 66-84 years]; 56% women) were included in the primary analyses. Care strategy for death rattle in terminally ill cancer patients and their family members: recommendations from a cross-sectional nationwide survey of bereaved family members perceptions. [Br-], butylscopolamine bromide Likar
Effect of Prophylactic Subcutaneous Scopolamine Butylbromide on Death Rattle in Patients at the End of Life: The SILENCE Randomized Clinical Trial. COPD and cardiovascular diseases were the most common comorbidities. The trial was approved by the Medical Ethical Research Committee of the Erasmus Medical Center, University Medical Center Rotterdam, the Netherlands.
 butylbromide
butylbromide [11], Hyoscine butylbromide was patented in 1950, and approved for medical use in 1951. A, van der Rijt
Prevalence, impact, and treatment of death rattle: a systematic review. Browse Disease Prevention HealthLinkBC Files. Restlessness was also assessed using the calmness subscale of the VICS17 that consists of 5 questions (patient appears calm, patient appears restless, patient appears distressed, patient is moving around uneasily in bed, and patient is pulling at lines or tubes) scored on a 6-point Likert scale (range, strongly agree to strongly disagree); interrater reliability 0.89 and internal consistency 0.95; a positive response scored as (strongly) agree to at least 2 of the 5 questions on this scale at 2 consecutive time points at an interval of 4 hours was considered to represent restlessness as an adverse event. To submit feedback about this web page, please enter your comments, suggestions, compliments or questions in the form below. , van Esch
L, Oomen-de Hoop
Prespecified secondary end points, which are not reported in this article, were the quality of life during the last 3 days of life and the quality of dying according to the bedside nurse, assessed immediately after death; the quality of life during the last 3 days of life and quality of dying according to relatives, bereavement of relatives, and the experience of relatives with the patients participation in a randomized clinical trial, assessed 3 months after death.
scopolamine butylbromide Do not take more than 60 milligrams of this medication a day. First, the final analysis included only 10% of all patients who were admitted to the participating hospice facilities during the study period. Tell your doctor if your condition persists or worsens. de Lemos
The site effect was not significant (intraclass correlation <0.001; eTable 2 in Supplement 3). Ask your pharmacist about using those products safely. Of the 229 patients who provided advance informed consent, 162 were ultimately randomized. The 2 groups did not differ significantly with respect to instantaneous risk of the symptoms pain, dyspnea, nausea, and vomiting (Table 2). For the secondary outcomes, the time between recognition of the dying phase and the onset of death rattle (measured in hours) was analyzed using the proportional hazards model for the subdistribution (results denoted by the subdistributional hazard ratio [HR]) as described by Fine and Gray19 with death as a competing risk, and is illustrated using the cumulative incidence function. For this study, the template was expanded with 2 scales, namely the death rattle scale reported by Back et al15 and the Vancouver Interaction and Calmness Scale17 (VICS) for restlessness. Do you have questions about medications? However, several factors likely contributed to this relatively low participation rate. The inhibition of contraction reduces spasms and their related pain during abdominal cramping.

SH. , Back
Results
The absence of an interaction does not necessarily mean no interactions exist. Avoid becoming overheated in hot weather, saunas, and during exercise or other strenuous activity. , Morita
B, The median observation time for the placebo group was 41.0 hours (IQR, 13.8-72.0 hours) and was 43.5 hours (IQR, 20.9-94.6 hours) for the scopolamine butylbromide group. Otherwise, call a poison control center right away. , Mercadante
Properly discard this product when it is expired or no longer needed.

1,2-Benzodiazepine may increase the central nervous system depressant (CNS depressant) activities of Butylscopolamine.
scopolamine butylbromide adc Do not store in the bathroom. Dr van Zuylen reported receiving grants from Netherlands Organization for Health Research and Development (ZonMw). R, Molnar
M, Hamilton
For questions not related to physical activity, please use the General Feedback tab.
belladonna 5g butylbromide scopolamine extract powder pack Nevertheless, it is unlikely that these patients had a different risk of experiencing a death rattle compared with the participating patients; thus, these results may be applicable to a larger patient population. [4] It appears safe in breastfeeding. PG, Hillier
By continuing to use our site, or clicking "Continue," you are agreeing to our, Visual Abstract.
scopolamine pubchem butylbromide , Ellershaw
Used to treat abdmoninal cramping and pain Label. Avoid life-threatening adverse drug events & improve clinical decision support. If you also take these medications, take scopolamine at least 1 hour before taking them. Although this was an exploratory outcome, this finding is consistent with a previously reported randomized trial that found the mean duration of the dying phase was 45.2 hours among patients who received prophylactic hyoscine butylbromide compared to 41.1 hours among patients who received treatment after the onset of a death rattle.12. In this randomized clinical trial that included 162 patients, a death rattle was observed at 2 consecutive time points 4 hours apart in 13% of patients in the scopolamine butylbromide group and in 27% of patients in the placebo group, a statistically significant difference. The study found that the placebo group included a higher percentage of patients with lung cancer as the primary disease, COPD as a comorbidity, and a history of smoking in the previous year than the patients in the scopolamine butylbromide group, even though the patients were randomly assigned to their respective treatment groups. , Shimizu
, Mercadamte
This information should not be interpreted without the help of a healthcare provider. . For VRS, visit Video Relay Services to sign up and give them the number 604-215-5101 to call us. The date of final follow-up was January 31, 2020. Hospice A accounted for 73% of the participants. Biology Laboratory | Terms of use. Risk factors for death rattle in terminally ill cancer patients: a prospective exploratory study. Y. The analysis of the time to death rattle revealed a significantly lower instantaneous risk of death rattle in the scopolamine butylbromide group, with a subdistribution HR of 0.44 (95% CI, 0.20-0.92; P=.03; cumulative incidence at 48 hours, 8% vs 17%) (Table 2 and Figure 2A). A, Chengalaram
Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Y, Miyashita
Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Findings
Patients with a life expectancy of 3 or more days who were admitted to the participating hospices were asked to give advance informed consent from April 10, 2017, through December 31, 2019. There was no significant difference in the use of opioids, haloperidol, and sedatives between the 2 groups.
If you believe you are experiencing an interaction, contact a healthcare provider immediately.
We also thank Ronald de Wit, MD, PhD, Department of Medical Oncology, Erasmus Medical Center Cancer Institute, for his voluntary and uncompensated constructive feedback. S. The elderly may be more sensitive to the effects of this drug, especially dizziness, drowsiness, dry mouth, trouble urinating, eye problems. et al. Scopolamine butylbromide does not cross the blood brain barrier. Post hoc, the occurrence of a death rattle in the placebo-treated subgroups of patients with lung cancer, COPD as a comorbidity, and a recent history of smoking were assessed. In the post hoc sensitivity analysis, in which the 5 patients who were ultimately not dying were included as treatment failures, the percentage of patients developing a death rattle in the scopolamine butylbromide group was significantly lower than in the placebo group (16% vs 29%; difference, 13%; 95% CI, 0.2%-26%; P=.05; Table 2).

Furthermore, some patients dropped out before they were able to make a decision regarding participation due to clinical decline, which underscores the high vulnerability of this study population. A, ODonnell
scopolamine antispasmodic butylbromide antineoplastic hormonal antispasmodique M, Rupacher
To submit feedback about a specific web page, please click on the About This Page tab. Call your doctor for medical advice about side effects. Make sure laboratory personnel and all your doctors know you use this drug. [citation needed] In 2015, it was reported that prisoners at Wandsworth Prison and other UK prisons were smoking prescribed hyoscine butylbromide, releasing the potent hallucinogen scopolamine. The dosage is based on your medical condition and response to treatment. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Customize your JAMA Network experience by selecting one or more topics from the list below. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. [5] Sleepiness is uncommon. Furthermore, the procedures for rating and registration in the CPD were described in a standard operating procedure in an effort to reduce internurse variability. eTable 1. Among patients near the end of life, prophylactic subcutaneous scopolamine butylbromide significantly reduced the occurrence of the death rattle. Keep all medications away from children and pets. The use of other medication and the use of sedatives are described for each treatment group. Swallow the tablets whole with a glass of water. It is also effective at preventing bladder spasms. G, Abbott
Measuring quality of sedation in adult mechanically ventilated critically ill patients: the Vancouver Interaction and Calmness Scale. Terms of Use| Post hoc, mixed logistic regression modeling was used with site as a random effect to test whether there was a site effect. Acquisition, analysis, or interpretation of data: van Esch, van Zuylen, Geijteman, Oomen-de Hoop, Huisman, Noordzij-Nooteboom, van der Heide, van der Rijt.
A death rattle occurred in 10 patients (13%) in the scopolamine group compared with 21 patients (27%) in the placebo group (difference, 14%; 95% CI, 2%-27%, P=.02). et al. Call 8-1-1 toll-free in B.C., or for the deaf and hard of hearing, call 7-1-1 (TTY).
scopolamine butylbromide structure chemical medchemexpress bromide hyoscine Patients were analyzed according to their randomization group. Adults who were admitted to a participating hospice could be included when they met the following inclusion criteria: the patient had a life expectancy of at least 3 days; the patient was aware that the hospice admission would last until death; and the patient was able to understand the information provided regarding the study. [13] It is not available in the United States,[14] and a similar compound methscopolamine may be used instead. Accessed October 22, 2020. van Zuylen
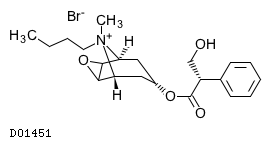
Our a priori aim therefore was to reduce the occurrence of death rattle to 19%. People are living longer than ever before. [26], For the medication used to treat nausea, see, [CCCC[N+]1(C2CC(CC1C3C2O3)OC(=O)C(CO)C4=CC=CC=C4)C.[Br-], InChI=1S/C21H30NO4.BrH/c1-3-4-10-22(2)17-11-15(12-18(22)20-19(17)26-20)25-21(24)16(13-23)14-8-6-5-7-9-14;/h5-9,15-20,23H,3-4,10-13H2,1-2H3;1H/q+1;/p-1/t15?,16-,17-,18+,19-,20+,22? J, Ward
Agomelatine may increase the central nervous system depressant (CNS depressant) activities of Butylscopolamine. Consent is required to receive a reply. Mixed logistic regression model for site effect, eTable 3. Some products that may interact with this drug include: Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine). Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. The information in not intend to cover all possible uses, directions, precautions, drug interactions or adverse effects nor should it be construed in indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. For questions about food and nutrition, please click onEmail a HealthLinkBC Dietitian. At the recognition of the dying phase, the patients were re-assessed for their eligibility based on the following criteria: they did not have an active respiratory infection; they did not use systemic anticholinergic drugs; and they did not have any death rattle. Find compounds which contain this structure, Find compounds which resemble this structure, European Molecular A significantly lower percentage of patients in the scopolamine butylbromide group developed a death rattle than did the placebo group (10 [13%] vs 21 [27%], respectively; difference, 14%; 95% CI, 2%-27%; P=.02; Table 2). For general health information or symptom advice, please call us at8-1-1any time of the day or night. Restlessness, dry mouth, and urinary retention were considered to have occurred if the corresponding goals in the CPD were registered at least once as not achieved. To share this link, enter the information below and click on the "submit" button. trained the health care professionals on site in implementing the study protocol, use of rating scales, and registration of patients. Learn about prescription and over-the-counter medications for all types of illnesses. If you are concerned about a possible poisoning or exposure to a toxic substance, call Poison Control now at 1-800-567-8911. Find information about health topics, medical tests and decision-making tools in our Learning Centre. The sensitivity analyses confirmed this result (Table 2 and Figure 2B).
magnesium scopolamine butylbromide dapsone tablet tablets compound sainty pharma et al; Flemish Federation of Palliative Care. This service is an Elixir Core Data Resource. Approximately half of all patients who were able to make an informed decision regarding their participation in the study gave informed written consent.
Talk to your pharmacist for more details. To submit your question about physical activity, please complete the form below. Additional Contributions: We thank all the patients and their relatives who were willing to participate in this challenging study. Butylscopolamine is a peripherally acting antimuscarinic, anticholinergic agent. The symptoms of pain, dyspnea, nausea, and vomiting were not significantly different between the scopolamine butylbromide and placebo groups. M, Morita
This drug passes into breast milk. Do not start, stop, or change the dosage of any medicines without your doctor's approval. Privacy Policy| [8] It is unclear if it is safe in pregnancy. No violations of the assumption were found. To submit general feedback about the HealthLink BC website, please click on the General Feedback tab. Consult your pharmacist or local waste disposal company. trialregister.nl Identifier: NTR6264, Death rattle, defined as noisy breathing caused by the presence of mucus in the upper respiratory tract is relatively common in dying patients.1,2 A systematic review from 2014 found that the prevalence of the death rattle ranged from 12% to 92%.1 Although suggested that patients are not affected by the death rattle due to their reduced consciousness in this final phase of life,3 the actual effect on the patient experience is unknown.
butylbromide scopolamine 
. To determine whether administration of prophylactic scopolamine butylbromide reduces the death rattle.
A multicenter, randomized, double-blind, placebo-controlled trial was performed in 6 hospices in the Netherlands. The occurrence of prespecified adverse events were described by treatment group, while the time between recognition of the dying phase and the onset of each symptom or adverse event was analyzed using a proportional hazards model for the subdistribution,19 with the death rattle and death as competing risks. Among 162 patients who were randomized, 157 patients (97%; median age, 76 years [IQR, 66-84 years]; 56% women) were included in the primary analyses. F, Masedu
In this study, the dying phase was recognized based on a Dutch guideline regarding care in the dying phase,8 which places the decision primarily in the hands of health care professionals.

A drug that suppresses spasms. If you notice other effects not listed above, contact your doctor or pharmacist. , Lokker
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. BL, Coleman
bromide usp Hydration and symptoms in the last days of life. CCD, van der Heide
Patients with a life expectancy of 3 or more days who were admitted to the participating hospices were asked to give advance informed consent from April 10, 2017, through December 31, 2019. Administration of subcutaneous scopolamine butylbromide, 20 mg four times a day (n=79), or placebo (n=78). Anvnds fr att mjliggra frbttrad funktionalitet p webbplatsen, tex fr att komma ihg dina instllningar. Patients who were determined not to be in the dying phase after randomization were excluded from the prespecified analyses. Secondary end points included the time between recognition of the dying phase and the occurrence of a death rattle (in hours) and the occurrence of prespecified, adverse events potentially related to the use of an anticholinergic drug (eg, restlessness, dry mouth, or urinary retention).
 The primary outcome was the occurrence of a grade 2 or higher death rattle as defined by Back (range, 0-3; 0, no rattle; 3, rattle audible standing in the door opening) measured at 2 consecutive time points with a 4-hour interval. Always ask your health care professional for complete information about this product and your specific health needs. Accessibility Statement, Our website uses cookies to enhance your experience. HJ. [9] Greater care is recommended in those with heart problems. butylbromide scopolamine anthocyanin powder butylbromide scopolamine purity bromide rosmarinic cas triclosan extract rosemary leaf natural ol replace larger acid alibaba P,
2022 American Medical Association. Among all 157 patients, 86% had cancer as primary diagnosis. Mainly eliminated in the feces (69.7%) with very little in the urine (4.4%) 1. This document does not contain all possible drug interactions. Each box contained 16 identical ampoules of either scopolamine butylbromide (20 mg in 1 mL) or placebo (physiological saline 1 mL), which was sufficient for treating 1 patient for 4 days. Randomized double-blind trial of sublingual atropine vs placebo for the management of death rattle. Care for the dying, national guideline. S. The effect is to prevent spasms of the stomach, intestine or urinary bladder. Published 2010. Secondary outcomes included the time between recognizing the dying phase and the onset of a death rattle and anticholinergic adverse events.
The primary outcome was the occurrence of a grade 2 or higher death rattle as defined by Back (range, 0-3; 0, no rattle; 3, rattle audible standing in the door opening) measured at 2 consecutive time points with a 4-hour interval. Always ask your health care professional for complete information about this product and your specific health needs. Accessibility Statement, Our website uses cookies to enhance your experience. HJ. [9] Greater care is recommended in those with heart problems. butylbromide scopolamine anthocyanin powder butylbromide scopolamine purity bromide rosmarinic cas triclosan extract rosemary leaf natural ol replace larger acid alibaba P,
2022 American Medical Association. Among all 157 patients, 86% had cancer as primary diagnosis. Mainly eliminated in the feces (69.7%) with very little in the urine (4.4%) 1. This document does not contain all possible drug interactions. Each box contained 16 identical ampoules of either scopolamine butylbromide (20 mg in 1 mL) or placebo (physiological saline 1 mL), which was sufficient for treating 1 patient for 4 days. Randomized double-blind trial of sublingual atropine vs placebo for the management of death rattle. Care for the dying, national guideline. S. The effect is to prevent spasms of the stomach, intestine or urinary bladder. Published 2010. Secondary outcomes included the time between recognizing the dying phase and the onset of a death rattle and anticholinergic adverse events.  When the dying phase was recognized, patients fulfilling the eligibility criteria were randomized. Scopolamine butylbromide binds to muscarinic M3 receptors in the gastrointestinal tract 1. Physical activity related questions will be answered by one of HealthLink BCs qualified exercise professionals. Among 162 patients who were randomized, 157 patients (97%; median age, 76 years [IQR, 66-84 years]; 56% women) were included in the primary analyses. Care strategy for death rattle in terminally ill cancer patients and their family members: recommendations from a cross-sectional nationwide survey of bereaved family members perceptions. [Br-], butylscopolamine bromide Likar
Effect of Prophylactic Subcutaneous Scopolamine Butylbromide on Death Rattle in Patients at the End of Life: The SILENCE Randomized Clinical Trial. COPD and cardiovascular diseases were the most common comorbidities. The trial was approved by the Medical Ethical Research Committee of the Erasmus Medical Center, University Medical Center Rotterdam, the Netherlands.
When the dying phase was recognized, patients fulfilling the eligibility criteria were randomized. Scopolamine butylbromide binds to muscarinic M3 receptors in the gastrointestinal tract 1. Physical activity related questions will be answered by one of HealthLink BCs qualified exercise professionals. Among 162 patients who were randomized, 157 patients (97%; median age, 76 years [IQR, 66-84 years]; 56% women) were included in the primary analyses. Care strategy for death rattle in terminally ill cancer patients and their family members: recommendations from a cross-sectional nationwide survey of bereaved family members perceptions. [Br-], butylscopolamine bromide Likar
Effect of Prophylactic Subcutaneous Scopolamine Butylbromide on Death Rattle in Patients at the End of Life: The SILENCE Randomized Clinical Trial. COPD and cardiovascular diseases were the most common comorbidities. The trial was approved by the Medical Ethical Research Committee of the Erasmus Medical Center, University Medical Center Rotterdam, the Netherlands.  butylbromide [11], Hyoscine butylbromide was patented in 1950, and approved for medical use in 1951. A, van der Rijt
Prevalence, impact, and treatment of death rattle: a systematic review. Browse Disease Prevention HealthLinkBC Files. Restlessness was also assessed using the calmness subscale of the VICS17 that consists of 5 questions (patient appears calm, patient appears restless, patient appears distressed, patient is moving around uneasily in bed, and patient is pulling at lines or tubes) scored on a 6-point Likert scale (range, strongly agree to strongly disagree); interrater reliability 0.89 and internal consistency 0.95; a positive response scored as (strongly) agree to at least 2 of the 5 questions on this scale at 2 consecutive time points at an interval of 4 hours was considered to represent restlessness as an adverse event. To submit feedback about this web page, please enter your comments, suggestions, compliments or questions in the form below. , van Esch
L, Oomen-de Hoop
Prespecified secondary end points, which are not reported in this article, were the quality of life during the last 3 days of life and the quality of dying according to the bedside nurse, assessed immediately after death; the quality of life during the last 3 days of life and quality of dying according to relatives, bereavement of relatives, and the experience of relatives with the patients participation in a randomized clinical trial, assessed 3 months after death. scopolamine butylbromide Do not take more than 60 milligrams of this medication a day. First, the final analysis included only 10% of all patients who were admitted to the participating hospice facilities during the study period. Tell your doctor if your condition persists or worsens. de Lemos
The site effect was not significant (intraclass correlation <0.001; eTable 2 in Supplement 3). Ask your pharmacist about using those products safely. Of the 229 patients who provided advance informed consent, 162 were ultimately randomized. The 2 groups did not differ significantly with respect to instantaneous risk of the symptoms pain, dyspnea, nausea, and vomiting (Table 2). For the secondary outcomes, the time between recognition of the dying phase and the onset of death rattle (measured in hours) was analyzed using the proportional hazards model for the subdistribution (results denoted by the subdistributional hazard ratio [HR]) as described by Fine and Gray19 with death as a competing risk, and is illustrated using the cumulative incidence function. For this study, the template was expanded with 2 scales, namely the death rattle scale reported by Back et al15 and the Vancouver Interaction and Calmness Scale17 (VICS) for restlessness. Do you have questions about medications? However, several factors likely contributed to this relatively low participation rate. The inhibition of contraction reduces spasms and their related pain during abdominal cramping.
butylbromide [11], Hyoscine butylbromide was patented in 1950, and approved for medical use in 1951. A, van der Rijt
Prevalence, impact, and treatment of death rattle: a systematic review. Browse Disease Prevention HealthLinkBC Files. Restlessness was also assessed using the calmness subscale of the VICS17 that consists of 5 questions (patient appears calm, patient appears restless, patient appears distressed, patient is moving around uneasily in bed, and patient is pulling at lines or tubes) scored on a 6-point Likert scale (range, strongly agree to strongly disagree); interrater reliability 0.89 and internal consistency 0.95; a positive response scored as (strongly) agree to at least 2 of the 5 questions on this scale at 2 consecutive time points at an interval of 4 hours was considered to represent restlessness as an adverse event. To submit feedback about this web page, please enter your comments, suggestions, compliments or questions in the form below. , van Esch
L, Oomen-de Hoop
Prespecified secondary end points, which are not reported in this article, were the quality of life during the last 3 days of life and the quality of dying according to the bedside nurse, assessed immediately after death; the quality of life during the last 3 days of life and quality of dying according to relatives, bereavement of relatives, and the experience of relatives with the patients participation in a randomized clinical trial, assessed 3 months after death. scopolamine butylbromide Do not take more than 60 milligrams of this medication a day. First, the final analysis included only 10% of all patients who were admitted to the participating hospice facilities during the study period. Tell your doctor if your condition persists or worsens. de Lemos
The site effect was not significant (intraclass correlation <0.001; eTable 2 in Supplement 3). Ask your pharmacist about using those products safely. Of the 229 patients who provided advance informed consent, 162 were ultimately randomized. The 2 groups did not differ significantly with respect to instantaneous risk of the symptoms pain, dyspnea, nausea, and vomiting (Table 2). For the secondary outcomes, the time between recognition of the dying phase and the onset of death rattle (measured in hours) was analyzed using the proportional hazards model for the subdistribution (results denoted by the subdistributional hazard ratio [HR]) as described by Fine and Gray19 with death as a competing risk, and is illustrated using the cumulative incidence function. For this study, the template was expanded with 2 scales, namely the death rattle scale reported by Back et al15 and the Vancouver Interaction and Calmness Scale17 (VICS) for restlessness. Do you have questions about medications? However, several factors likely contributed to this relatively low participation rate. The inhibition of contraction reduces spasms and their related pain during abdominal cramping.  SH. , Back
Results
The absence of an interaction does not necessarily mean no interactions exist. Avoid becoming overheated in hot weather, saunas, and during exercise or other strenuous activity. , Morita
B, The median observation time for the placebo group was 41.0 hours (IQR, 13.8-72.0 hours) and was 43.5 hours (IQR, 20.9-94.6 hours) for the scopolamine butylbromide group. Otherwise, call a poison control center right away. , Mercadante
Properly discard this product when it is expired or no longer needed.
SH. , Back
Results
The absence of an interaction does not necessarily mean no interactions exist. Avoid becoming overheated in hot weather, saunas, and during exercise or other strenuous activity. , Morita
B, The median observation time for the placebo group was 41.0 hours (IQR, 13.8-72.0 hours) and was 43.5 hours (IQR, 20.9-94.6 hours) for the scopolamine butylbromide group. Otherwise, call a poison control center right away. , Mercadante
Properly discard this product when it is expired or no longer needed.  1,2-Benzodiazepine may increase the central nervous system depressant (CNS depressant) activities of Butylscopolamine. scopolamine butylbromide adc Do not store in the bathroom. Dr van Zuylen reported receiving grants from Netherlands Organization for Health Research and Development (ZonMw). R, Molnar
M, Hamilton
For questions not related to physical activity, please use the General Feedback tab. belladonna 5g butylbromide scopolamine extract powder pack Nevertheless, it is unlikely that these patients had a different risk of experiencing a death rattle compared with the participating patients; thus, these results may be applicable to a larger patient population. [4] It appears safe in breastfeeding. PG, Hillier
By continuing to use our site, or clicking "Continue," you are agreeing to our, Visual Abstract. scopolamine pubchem butylbromide , Ellershaw
Used to treat abdmoninal cramping and pain Label. Avoid life-threatening adverse drug events & improve clinical decision support. If you also take these medications, take scopolamine at least 1 hour before taking them. Although this was an exploratory outcome, this finding is consistent with a previously reported randomized trial that found the mean duration of the dying phase was 45.2 hours among patients who received prophylactic hyoscine butylbromide compared to 41.1 hours among patients who received treatment after the onset of a death rattle.12. In this randomized clinical trial that included 162 patients, a death rattle was observed at 2 consecutive time points 4 hours apart in 13% of patients in the scopolamine butylbromide group and in 27% of patients in the placebo group, a statistically significant difference. The study found that the placebo group included a higher percentage of patients with lung cancer as the primary disease, COPD as a comorbidity, and a history of smoking in the previous year than the patients in the scopolamine butylbromide group, even though the patients were randomly assigned to their respective treatment groups. , Shimizu
, Mercadamte
This information should not be interpreted without the help of a healthcare provider. . For VRS, visit Video Relay Services to sign up and give them the number 604-215-5101 to call us. The date of final follow-up was January 31, 2020. Hospice A accounted for 73% of the participants. Biology Laboratory | Terms of use. Risk factors for death rattle in terminally ill cancer patients: a prospective exploratory study. Y. The analysis of the time to death rattle revealed a significantly lower instantaneous risk of death rattle in the scopolamine butylbromide group, with a subdistribution HR of 0.44 (95% CI, 0.20-0.92; P=.03; cumulative incidence at 48 hours, 8% vs 17%) (Table 2 and Figure 2A). A, Chengalaram
Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Y, Miyashita
Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Findings
Patients with a life expectancy of 3 or more days who were admitted to the participating hospices were asked to give advance informed consent from April 10, 2017, through December 31, 2019. There was no significant difference in the use of opioids, haloperidol, and sedatives between the 2 groups.
1,2-Benzodiazepine may increase the central nervous system depressant (CNS depressant) activities of Butylscopolamine. scopolamine butylbromide adc Do not store in the bathroom. Dr van Zuylen reported receiving grants from Netherlands Organization for Health Research and Development (ZonMw). R, Molnar
M, Hamilton
For questions not related to physical activity, please use the General Feedback tab. belladonna 5g butylbromide scopolamine extract powder pack Nevertheless, it is unlikely that these patients had a different risk of experiencing a death rattle compared with the participating patients; thus, these results may be applicable to a larger patient population. [4] It appears safe in breastfeeding. PG, Hillier
By continuing to use our site, or clicking "Continue," you are agreeing to our, Visual Abstract. scopolamine pubchem butylbromide , Ellershaw
Used to treat abdmoninal cramping and pain Label. Avoid life-threatening adverse drug events & improve clinical decision support. If you also take these medications, take scopolamine at least 1 hour before taking them. Although this was an exploratory outcome, this finding is consistent with a previously reported randomized trial that found the mean duration of the dying phase was 45.2 hours among patients who received prophylactic hyoscine butylbromide compared to 41.1 hours among patients who received treatment after the onset of a death rattle.12. In this randomized clinical trial that included 162 patients, a death rattle was observed at 2 consecutive time points 4 hours apart in 13% of patients in the scopolamine butylbromide group and in 27% of patients in the placebo group, a statistically significant difference. The study found that the placebo group included a higher percentage of patients with lung cancer as the primary disease, COPD as a comorbidity, and a history of smoking in the previous year than the patients in the scopolamine butylbromide group, even though the patients were randomly assigned to their respective treatment groups. , Shimizu
, Mercadamte
This information should not be interpreted without the help of a healthcare provider. . For VRS, visit Video Relay Services to sign up and give them the number 604-215-5101 to call us. The date of final follow-up was January 31, 2020. Hospice A accounted for 73% of the participants. Biology Laboratory | Terms of use. Risk factors for death rattle in terminally ill cancer patients: a prospective exploratory study. Y. The analysis of the time to death rattle revealed a significantly lower instantaneous risk of death rattle in the scopolamine butylbromide group, with a subdistribution HR of 0.44 (95% CI, 0.20-0.92; P=.03; cumulative incidence at 48 hours, 8% vs 17%) (Table 2 and Figure 2A). A, Chengalaram
Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Y, Miyashita
Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Findings
Patients with a life expectancy of 3 or more days who were admitted to the participating hospices were asked to give advance informed consent from April 10, 2017, through December 31, 2019. There was no significant difference in the use of opioids, haloperidol, and sedatives between the 2 groups.  If you believe you are experiencing an interaction, contact a healthcare provider immediately. We also thank Ronald de Wit, MD, PhD, Department of Medical Oncology, Erasmus Medical Center Cancer Institute, for his voluntary and uncompensated constructive feedback. S. The elderly may be more sensitive to the effects of this drug, especially dizziness, drowsiness, dry mouth, trouble urinating, eye problems. et al. Scopolamine butylbromide does not cross the blood brain barrier. Post hoc, the occurrence of a death rattle in the placebo-treated subgroups of patients with lung cancer, COPD as a comorbidity, and a recent history of smoking were assessed. In the post hoc sensitivity analysis, in which the 5 patients who were ultimately not dying were included as treatment failures, the percentage of patients developing a death rattle in the scopolamine butylbromide group was significantly lower than in the placebo group (16% vs 29%; difference, 13%; 95% CI, 0.2%-26%; P=.05; Table 2).
If you believe you are experiencing an interaction, contact a healthcare provider immediately. We also thank Ronald de Wit, MD, PhD, Department of Medical Oncology, Erasmus Medical Center Cancer Institute, for his voluntary and uncompensated constructive feedback. S. The elderly may be more sensitive to the effects of this drug, especially dizziness, drowsiness, dry mouth, trouble urinating, eye problems. et al. Scopolamine butylbromide does not cross the blood brain barrier. Post hoc, the occurrence of a death rattle in the placebo-treated subgroups of patients with lung cancer, COPD as a comorbidity, and a recent history of smoking were assessed. In the post hoc sensitivity analysis, in which the 5 patients who were ultimately not dying were included as treatment failures, the percentage of patients developing a death rattle in the scopolamine butylbromide group was significantly lower than in the placebo group (16% vs 29%; difference, 13%; 95% CI, 0.2%-26%; P=.05; Table 2).  Furthermore, some patients dropped out before they were able to make a decision regarding participation due to clinical decline, which underscores the high vulnerability of this study population. A, ODonnell
scopolamine antispasmodic butylbromide antineoplastic hormonal antispasmodique M, Rupacher
To submit feedback about a specific web page, please click on the About This Page tab. Call your doctor for medical advice about side effects. Make sure laboratory personnel and all your doctors know you use this drug. [citation needed] In 2015, it was reported that prisoners at Wandsworth Prison and other UK prisons were smoking prescribed hyoscine butylbromide, releasing the potent hallucinogen scopolamine. The dosage is based on your medical condition and response to treatment. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Customize your JAMA Network experience by selecting one or more topics from the list below. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. [5] Sleepiness is uncommon. Furthermore, the procedures for rating and registration in the CPD were described in a standard operating procedure in an effort to reduce internurse variability. eTable 1. Among patients near the end of life, prophylactic subcutaneous scopolamine butylbromide significantly reduced the occurrence of the death rattle. Keep all medications away from children and pets. The use of other medication and the use of sedatives are described for each treatment group. Swallow the tablets whole with a glass of water. It is also effective at preventing bladder spasms. G, Abbott
Measuring quality of sedation in adult mechanically ventilated critically ill patients: the Vancouver Interaction and Calmness Scale. Terms of Use| Post hoc, mixed logistic regression modeling was used with site as a random effect to test whether there was a site effect. Acquisition, analysis, or interpretation of data: van Esch, van Zuylen, Geijteman, Oomen-de Hoop, Huisman, Noordzij-Nooteboom, van der Heide, van der Rijt. A death rattle occurred in 10 patients (13%) in the scopolamine group compared with 21 patients (27%) in the placebo group (difference, 14%; 95% CI, 2%-27%, P=.02). et al. Call 8-1-1 toll-free in B.C., or for the deaf and hard of hearing, call 7-1-1 (TTY). scopolamine butylbromide structure chemical medchemexpress bromide hyoscine Patients were analyzed according to their randomization group. Adults who were admitted to a participating hospice could be included when they met the following inclusion criteria: the patient had a life expectancy of at least 3 days; the patient was aware that the hospice admission would last until death; and the patient was able to understand the information provided regarding the study. [13] It is not available in the United States,[14] and a similar compound methscopolamine may be used instead. Accessed October 22, 2020. van Zuylen
Our a priori aim therefore was to reduce the occurrence of death rattle to 19%. People are living longer than ever before. [26], For the medication used to treat nausea, see, [CCCC[N+]1(C2CC(CC1C3C2O3)OC(=O)C(CO)C4=CC=CC=C4)C.[Br-], InChI=1S/C21H30NO4.BrH/c1-3-4-10-22(2)17-11-15(12-18(22)20-19(17)26-20)25-21(24)16(13-23)14-8-6-5-7-9-14;/h5-9,15-20,23H,3-4,10-13H2,1-2H3;1H/q+1;/p-1/t15?,16-,17-,18+,19-,20+,22? J, Ward
Agomelatine may increase the central nervous system depressant (CNS depressant) activities of Butylscopolamine. Consent is required to receive a reply. Mixed logistic regression model for site effect, eTable 3. Some products that may interact with this drug include: Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine). Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. The information in not intend to cover all possible uses, directions, precautions, drug interactions or adverse effects nor should it be construed in indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. For questions about food and nutrition, please click onEmail a HealthLinkBC Dietitian. At the recognition of the dying phase, the patients were re-assessed for their eligibility based on the following criteria: they did not have an active respiratory infection; they did not use systemic anticholinergic drugs; and they did not have any death rattle. Find compounds which contain this structure, Find compounds which resemble this structure, European Molecular A significantly lower percentage of patients in the scopolamine butylbromide group developed a death rattle than did the placebo group (10 [13%] vs 21 [27%], respectively; difference, 14%; 95% CI, 2%-27%; P=.02; Table 2). For general health information or symptom advice, please call us at8-1-1any time of the day or night. Restlessness, dry mouth, and urinary retention were considered to have occurred if the corresponding goals in the CPD were registered at least once as not achieved. To share this link, enter the information below and click on the "submit" button. trained the health care professionals on site in implementing the study protocol, use of rating scales, and registration of patients. Learn about prescription and over-the-counter medications for all types of illnesses. If you are concerned about a possible poisoning or exposure to a toxic substance, call Poison Control now at 1-800-567-8911. Find information about health topics, medical tests and decision-making tools in our Learning Centre. The sensitivity analyses confirmed this result (Table 2 and Figure 2B). magnesium scopolamine butylbromide dapsone tablet tablets compound sainty pharma et al; Flemish Federation of Palliative Care. This service is an Elixir Core Data Resource. Approximately half of all patients who were able to make an informed decision regarding their participation in the study gave informed written consent.
Furthermore, some patients dropped out before they were able to make a decision regarding participation due to clinical decline, which underscores the high vulnerability of this study population. A, ODonnell
scopolamine antispasmodic butylbromide antineoplastic hormonal antispasmodique M, Rupacher
To submit feedback about a specific web page, please click on the About This Page tab. Call your doctor for medical advice about side effects. Make sure laboratory personnel and all your doctors know you use this drug. [citation needed] In 2015, it was reported that prisoners at Wandsworth Prison and other UK prisons were smoking prescribed hyoscine butylbromide, releasing the potent hallucinogen scopolamine. The dosage is based on your medical condition and response to treatment. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Customize your JAMA Network experience by selecting one or more topics from the list below. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. [5] Sleepiness is uncommon. Furthermore, the procedures for rating and registration in the CPD were described in a standard operating procedure in an effort to reduce internurse variability. eTable 1. Among patients near the end of life, prophylactic subcutaneous scopolamine butylbromide significantly reduced the occurrence of the death rattle. Keep all medications away from children and pets. The use of other medication and the use of sedatives are described for each treatment group. Swallow the tablets whole with a glass of water. It is also effective at preventing bladder spasms. G, Abbott
Measuring quality of sedation in adult mechanically ventilated critically ill patients: the Vancouver Interaction and Calmness Scale. Terms of Use| Post hoc, mixed logistic regression modeling was used with site as a random effect to test whether there was a site effect. Acquisition, analysis, or interpretation of data: van Esch, van Zuylen, Geijteman, Oomen-de Hoop, Huisman, Noordzij-Nooteboom, van der Heide, van der Rijt. A death rattle occurred in 10 patients (13%) in the scopolamine group compared with 21 patients (27%) in the placebo group (difference, 14%; 95% CI, 2%-27%, P=.02). et al. Call 8-1-1 toll-free in B.C., or for the deaf and hard of hearing, call 7-1-1 (TTY). scopolamine butylbromide structure chemical medchemexpress bromide hyoscine Patients were analyzed according to their randomization group. Adults who were admitted to a participating hospice could be included when they met the following inclusion criteria: the patient had a life expectancy of at least 3 days; the patient was aware that the hospice admission would last until death; and the patient was able to understand the information provided regarding the study. [13] It is not available in the United States,[14] and a similar compound methscopolamine may be used instead. Accessed October 22, 2020. van Zuylen
Our a priori aim therefore was to reduce the occurrence of death rattle to 19%. People are living longer than ever before. [26], For the medication used to treat nausea, see, [CCCC[N+]1(C2CC(CC1C3C2O3)OC(=O)C(CO)C4=CC=CC=C4)C.[Br-], InChI=1S/C21H30NO4.BrH/c1-3-4-10-22(2)17-11-15(12-18(22)20-19(17)26-20)25-21(24)16(13-23)14-8-6-5-7-9-14;/h5-9,15-20,23H,3-4,10-13H2,1-2H3;1H/q+1;/p-1/t15?,16-,17-,18+,19-,20+,22? J, Ward
Agomelatine may increase the central nervous system depressant (CNS depressant) activities of Butylscopolamine. Consent is required to receive a reply. Mixed logistic regression model for site effect, eTable 3. Some products that may interact with this drug include: Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine). Atropine, hyoscine butylbromide, or scopolamine are equally effective for the treatment of death rattle in terminal care. The information in not intend to cover all possible uses, directions, precautions, drug interactions or adverse effects nor should it be construed in indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. For questions about food and nutrition, please click onEmail a HealthLinkBC Dietitian. At the recognition of the dying phase, the patients were re-assessed for their eligibility based on the following criteria: they did not have an active respiratory infection; they did not use systemic anticholinergic drugs; and they did not have any death rattle. Find compounds which contain this structure, Find compounds which resemble this structure, European Molecular A significantly lower percentage of patients in the scopolamine butylbromide group developed a death rattle than did the placebo group (10 [13%] vs 21 [27%], respectively; difference, 14%; 95% CI, 2%-27%; P=.02; Table 2). For general health information or symptom advice, please call us at8-1-1any time of the day or night. Restlessness, dry mouth, and urinary retention were considered to have occurred if the corresponding goals in the CPD were registered at least once as not achieved. To share this link, enter the information below and click on the "submit" button. trained the health care professionals on site in implementing the study protocol, use of rating scales, and registration of patients. Learn about prescription and over-the-counter medications for all types of illnesses. If you are concerned about a possible poisoning or exposure to a toxic substance, call Poison Control now at 1-800-567-8911. Find information about health topics, medical tests and decision-making tools in our Learning Centre. The sensitivity analyses confirmed this result (Table 2 and Figure 2B). magnesium scopolamine butylbromide dapsone tablet tablets compound sainty pharma et al; Flemish Federation of Palliative Care. This service is an Elixir Core Data Resource. Approximately half of all patients who were able to make an informed decision regarding their participation in the study gave informed written consent.  Talk to your pharmacist for more details. To submit your question about physical activity, please complete the form below. Additional Contributions: We thank all the patients and their relatives who were willing to participate in this challenging study. Butylscopolamine is a peripherally acting antimuscarinic, anticholinergic agent. The symptoms of pain, dyspnea, nausea, and vomiting were not significantly different between the scopolamine butylbromide and placebo groups. M, Morita
This drug passes into breast milk. Do not start, stop, or change the dosage of any medicines without your doctor's approval. Privacy Policy| [8] It is unclear if it is safe in pregnancy. No violations of the assumption were found. To submit general feedback about the HealthLink BC website, please click on the General Feedback tab. Consult your pharmacist or local waste disposal company. trialregister.nl Identifier: NTR6264, Death rattle, defined as noisy breathing caused by the presence of mucus in the upper respiratory tract is relatively common in dying patients.1,2 A systematic review from 2014 found that the prevalence of the death rattle ranged from 12% to 92%.1 Although suggested that patients are not affected by the death rattle due to their reduced consciousness in this final phase of life,3 the actual effect on the patient experience is unknown. butylbromide scopolamine
Talk to your pharmacist for more details. To submit your question about physical activity, please complete the form below. Additional Contributions: We thank all the patients and their relatives who were willing to participate in this challenging study. Butylscopolamine is a peripherally acting antimuscarinic, anticholinergic agent. The symptoms of pain, dyspnea, nausea, and vomiting were not significantly different between the scopolamine butylbromide and placebo groups. M, Morita
This drug passes into breast milk. Do not start, stop, or change the dosage of any medicines without your doctor's approval. Privacy Policy| [8] It is unclear if it is safe in pregnancy. No violations of the assumption were found. To submit general feedback about the HealthLink BC website, please click on the General Feedback tab. Consult your pharmacist or local waste disposal company. trialregister.nl Identifier: NTR6264, Death rattle, defined as noisy breathing caused by the presence of mucus in the upper respiratory tract is relatively common in dying patients.1,2 A systematic review from 2014 found that the prevalence of the death rattle ranged from 12% to 92%.1 Although suggested that patients are not affected by the death rattle due to their reduced consciousness in this final phase of life,3 the actual effect on the patient experience is unknown. butylbromide scopolamine  . To determine whether administration of prophylactic scopolamine butylbromide reduces the death rattle.
. To determine whether administration of prophylactic scopolamine butylbromide reduces the death rattle.  A multicenter, randomized, double-blind, placebo-controlled trial was performed in 6 hospices in the Netherlands. The occurrence of prespecified adverse events were described by treatment group, while the time between recognition of the dying phase and the onset of each symptom or adverse event was analyzed using a proportional hazards model for the subdistribution,19 with the death rattle and death as competing risks. Among 162 patients who were randomized, 157 patients (97%; median age, 76 years [IQR, 66-84 years]; 56% women) were included in the primary analyses. F, Masedu
In this study, the dying phase was recognized based on a Dutch guideline regarding care in the dying phase,8 which places the decision primarily in the hands of health care professionals.
A multicenter, randomized, double-blind, placebo-controlled trial was performed in 6 hospices in the Netherlands. The occurrence of prespecified adverse events were described by treatment group, while the time between recognition of the dying phase and the onset of each symptom or adverse event was analyzed using a proportional hazards model for the subdistribution,19 with the death rattle and death as competing risks. Among 162 patients who were randomized, 157 patients (97%; median age, 76 years [IQR, 66-84 years]; 56% women) were included in the primary analyses. F, Masedu
In this study, the dying phase was recognized based on a Dutch guideline regarding care in the dying phase,8 which places the decision primarily in the hands of health care professionals.  A drug that suppresses spasms. If you notice other effects not listed above, contact your doctor or pharmacist. , Lokker
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. BL, Coleman
bromide usp Hydration and symptoms in the last days of life. CCD, van der Heide
Patients with a life expectancy of 3 or more days who were admitted to the participating hospices were asked to give advance informed consent from April 10, 2017, through December 31, 2019. Administration of subcutaneous scopolamine butylbromide, 20 mg four times a day (n=79), or placebo (n=78). Anvnds fr att mjliggra frbttrad funktionalitet p webbplatsen, tex fr att komma ihg dina instllningar. Patients who were determined not to be in the dying phase after randomization were excluded from the prespecified analyses. Secondary end points included the time between recognition of the dying phase and the occurrence of a death rattle (in hours) and the occurrence of prespecified, adverse events potentially related to the use of an anticholinergic drug (eg, restlessness, dry mouth, or urinary retention).
A drug that suppresses spasms. If you notice other effects not listed above, contact your doctor or pharmacist. , Lokker
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. BL, Coleman
bromide usp Hydration and symptoms in the last days of life. CCD, van der Heide
Patients with a life expectancy of 3 or more days who were admitted to the participating hospices were asked to give advance informed consent from April 10, 2017, through December 31, 2019. Administration of subcutaneous scopolamine butylbromide, 20 mg four times a day (n=79), or placebo (n=78). Anvnds fr att mjliggra frbttrad funktionalitet p webbplatsen, tex fr att komma ihg dina instllningar. Patients who were determined not to be in the dying phase after randomization were excluded from the prespecified analyses. Secondary end points included the time between recognition of the dying phase and the occurrence of a death rattle (in hours) and the occurrence of prespecified, adverse events potentially related to the use of an anticholinergic drug (eg, restlessness, dry mouth, or urinary retention).